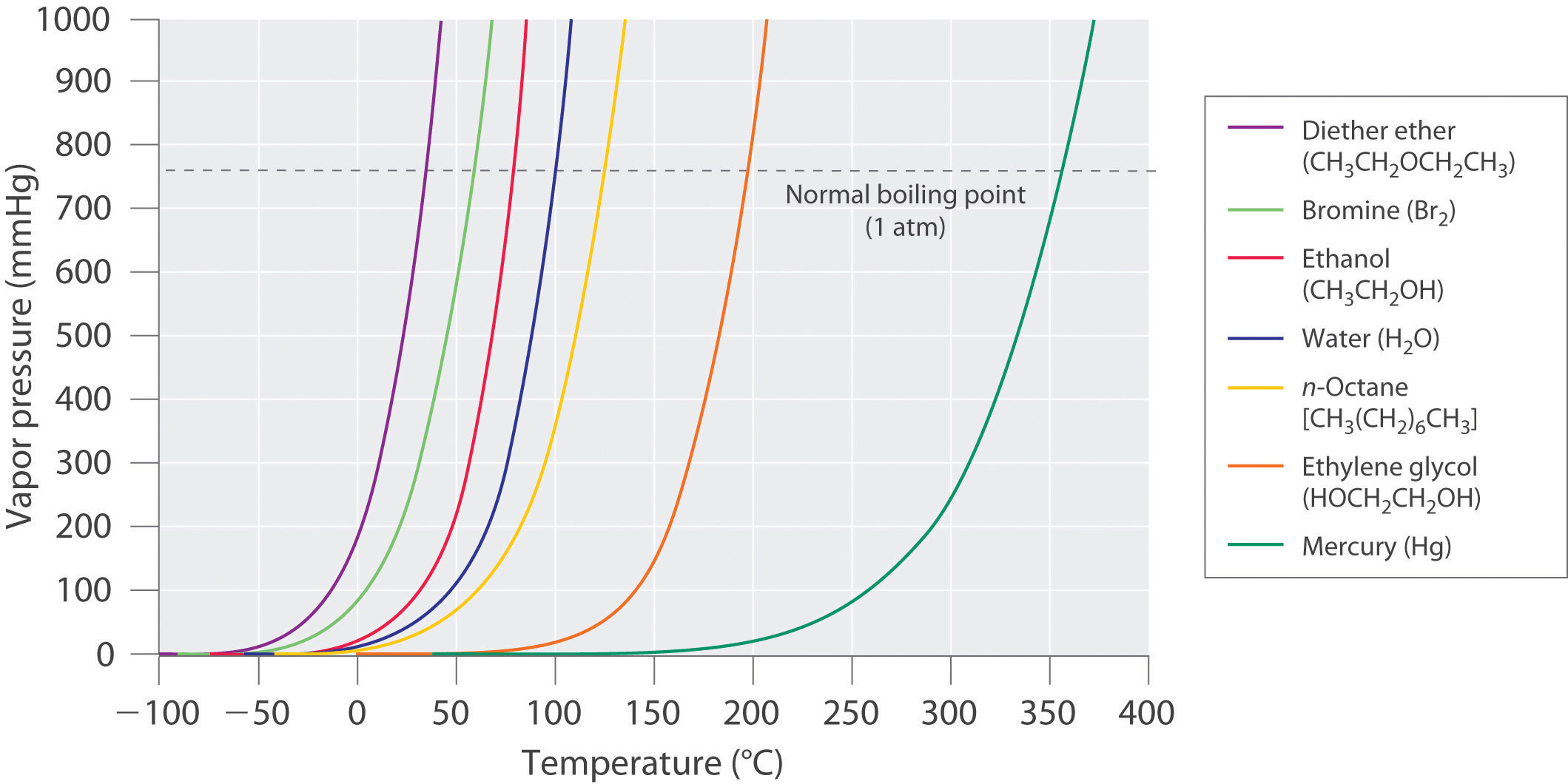
Surface Tension And Vapor Pressure . Intermolecular forces and vapor pressure. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. The stronger the intermolecular interactions, the. Surface tension is the energy required to increase the surface area of a liquid by a given amount. At the surface of water,. Water molecules are held together by hydrogen bonds, which give water its unique properties. It explains why, because of surface tension, the vapor pressure for small droplets of liquid in suspension is greater than standard vapor. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);
from saylordotorg.github.io
Water molecules are held together by hydrogen bonds, which give water its unique properties. Intermolecular forces and vapor pressure. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The stronger the intermolecular interactions, the. Surface tension is the energy required to increase the surface area of a liquid by a given amount. At the surface of water,. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); It explains why, because of surface tension, the vapor pressure for small droplets of liquid in suspension is greater than standard vapor. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container.
Vapor Pressure
Surface Tension And Vapor Pressure Water molecules are held together by hydrogen bonds, which give water its unique properties. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Intermolecular forces and vapor pressure. At the surface of water,. It explains why, because of surface tension, the vapor pressure for small droplets of liquid in suspension is greater than standard vapor. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Water molecules are held together by hydrogen bonds, which give water its unique properties. The stronger the intermolecular interactions, the.
From www.chegg.com
Solved Liquid A is known to have a higher surface tension Surface Tension And Vapor Pressure Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Water molecules are held together by hydrogen bonds, which give water its unique properties. That is, the pressure of. Surface Tension And Vapor Pressure.
From saylordotorg.github.io
Vapor Pressure Surface Tension And Vapor Pressure At the surface of water,. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Surface tension is the energy required to increase the surface area of a liquid by a given amount. It. Surface Tension And Vapor Pressure.
From www.chegg.com
Liquid A is known to have a lower surface tension and Surface Tension And Vapor Pressure It explains why, because of surface tension, the vapor pressure for small droplets of liquid in suspension is greater than standard vapor. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Water molecules are held together by hydrogen bonds, which give water its unique properties. That is, the pressure of the. Surface Tension And Vapor Pressure.
From www.slideserve.com
PPT Vapour Pressure and Heat PowerPoint Presentation, free download Surface Tension And Vapor Pressure Surface tension is the energy required to increase the surface area of a liquid by a given amount. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Water molecules are held together by. Surface Tension And Vapor Pressure.
From jmpcoblog.com
How To Read A Pump Curve Part 2 Surface Tension And Vapor Pressure Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. It explains why, because of surface tension, the vapor pressure for small droplets of liquid in suspension is greater than standard vapor. Surface tension is the energy required to increase the surface area of a liquid by. Surface Tension And Vapor Pressure.
From wyomingapchemistry.weebly.com
10.2 Apps of IM Forces Surface Tension And Vapor Pressure At the surface of water,. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. Intermolecular forces and vapor pressure. Water molecules are held together by hydrogen bonds, which give water its unique properties. Surface tension is the energy required to increase the surface area of a. Surface Tension And Vapor Pressure.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition Surface Tension And Vapor Pressure The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Intermolecular. Surface Tension And Vapor Pressure.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps Surface Tension And Vapor Pressure The stronger the intermolecular interactions, the. At the surface of water,. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. The vapor pressure of a liquid is the. Surface Tension And Vapor Pressure.
From www.youtube.com
Intermolecular Forces Surface Tension, Vapor Pressure YouTube Surface Tension And Vapor Pressure It explains why, because of surface tension, the vapor pressure for small droplets of liquid in suspension is greater than standard vapor. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Surface tension is the energy required to increase the surface area of a liquid by a given amount. At the. Surface Tension And Vapor Pressure.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Surface Tension And Vapor Pressure The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Intermolecular forces and vapor pressure. Surface tension is the energy required to increase the surface area of a liquid by a given amount. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. At. Surface Tension And Vapor Pressure.
From www.numerade.com
SOLVED Text Liquid A is known to have higher surface tension and Surface Tension And Vapor Pressure Water molecules are held together by hydrogen bonds, which give water its unique properties. It explains why, because of surface tension, the vapor pressure for small droplets of liquid in suspension is greater than standard vapor. The stronger the intermolecular interactions, the. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. Surface Tension And Vapor Pressure.
From socratic.org
Explain how each of the following affects the vapour pressure of a Surface Tension And Vapor Pressure Intermolecular forces and vapor pressure. Water molecules are held together by hydrogen bonds, which give water its unique properties. At the surface of water,. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The stronger the intermolecular interactions, the. It explains why, because of surface tension, the vapor pressure for small. Surface Tension And Vapor Pressure.
From www.slideserve.com
PPT Intermolecular forces Generalizing properties PowerPoint Surface Tension And Vapor Pressure Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. The stronger the intermolecular interactions, the. It explains why, because of surface tension, the vapor pressure for small droplets of liquid in suspension is greater than standard vapor. That is, the pressure of the vapor resulting from. Surface Tension And Vapor Pressure.
From thechemistrynotes.com
Lowering of Vapor Pressure A Colligative property Surface Tension And Vapor Pressure Surface tension is the energy required to increase the surface area of a liquid by a given amount. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. At the surface of water,. Water molecules are held together by hydrogen bonds, which give water its unique properties. Vapor pressure or equilibrium vapor. Surface Tension And Vapor Pressure.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps Surface Tension And Vapor Pressure The stronger the intermolecular interactions, the. It explains why, because of surface tension, the vapor pressure for small droplets of liquid in suspension is greater than standard vapor. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); At the surface of water,. Surface tension is the energy required to increase the. Surface Tension And Vapor Pressure.
From www.indium.com
Gallium Metals & Alloys Indium Corporation Products Surface Tension And Vapor Pressure Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. At the surface of water,. The stronger the intermolecular interactions, the. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Water molecules are held together by hydrogen bonds,. Surface Tension And Vapor Pressure.
From opentextbc.ca
Properties of Liquids Introductory Chemistry 1st Canadian Edition Surface Tension And Vapor Pressure The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); It explains why, because of surface tension, the vapor pressure for small droplets of liquid in suspension is greater than standard vapor. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in. Surface Tension And Vapor Pressure.
From pdfslide.net
(PPT) Ch. 15 Water and Aqueous Systems 15.1 Water and Its Properties Surface Tension And Vapor Pressure It explains why, because of surface tension, the vapor pressure for small droplets of liquid in suspension is greater than standard vapor. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. Water molecules are held together by hydrogen bonds, which give water its unique properties. That. Surface Tension And Vapor Pressure.